You are viewing this post: Exotoxin and Endotoxin DR ABDELRAHAMAN ATTIYA | endotoxin คือ
Table of Contents
Exotoxin and Endotoxin DR ABDELRAHAMAN ATTIYA
นอกจากการดูบทความนี้แล้ว คุณยังสามารถดูข้อมูลที่เป็นประโยชน์อื่นๆ อีกมากมายที่เราให้ไว้ที่นี่: ดูเพิ่มเติม
LAL test for endotoxin
LAL limulus amebocyte lysate
LAL test for endotoxin
Twitter @chemfunman
Subscribe now to learn more about science and how the brain works.
Credit: Rachel Lim, in collaboration with Fun Man
Lab credit: Prof. Sam Li Fong Yau
Reference Paper: 10.1038/s4159801711232x
VIDEOS ON CHEMISTRY TECHNIQUES IN THE LAB
Schlenk Line
https://www.youtube.com/watch?v=Eov60kI7yw8
ChemDraw Pro 15.0 Tutorial
https://www.youtube.com/watch?v=037WCSsoivo

Lipopolysaccharides | LPS | Endotoxin | Bacterial toxin | Inflammation | Basic Science Series
Lipopolysaccharides | LPS | Endotoxin | Bacterial toxin | Inflammation | Basic Science Series
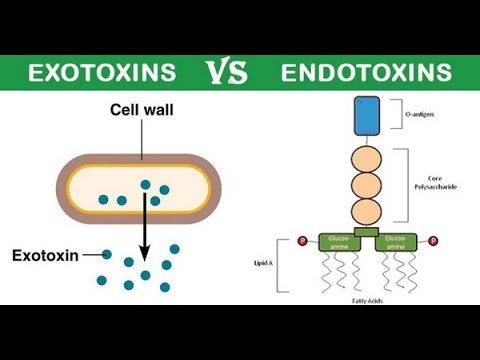
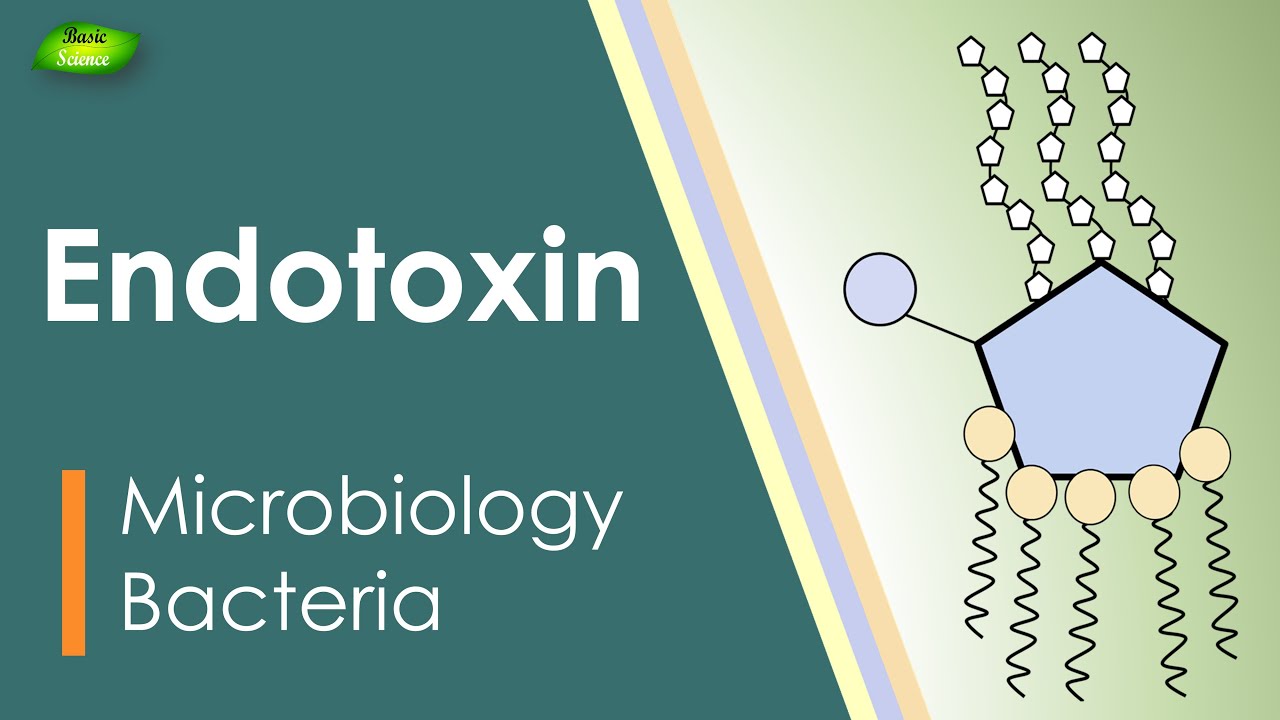
Lipopolysaccharides (LPS), also known as lipoglycans and endotoxins. They are called lipoglycans because they are consist of a lipid and a polysaccharide component. They are called endotoxin because they are present in the bacterial cell wall (endo) and are extremely very toxic to the human body.
• The toxic activity of bacterial LPS was first discovered and termed \”endotoxin\” by Richard Friedrich Johannes Pfeiffer. Who distinguished between exotoxins and endotoxin. The exotoxin is the toxin that is released by bacteria into the surrounding environment, and endotoxins, which he considered to be a toxin kept \”within\” the bacterial cell and released only after the destruction of the bacterial cell wall. Subsequent work showed that the release of LPS from gramnegative microbes does not necessarily require the destruction of the bacterial cell wall, but rather, LPS is secreted as part of the normal physiological activity of membrane vesicle trafficking in the form of bacterial outer membrane vesicles (OMVs).
•
• LPS is the major component of the outer membrane of Gramnegative bacteria, contributing greatly to the structural integrity of the bacteria.
• LPS protects the membrane from certain kinds of chemical attacks. LPS also increases the negative charge of the cell membrane and helps stabilize the overall membrane structure.
• LPS induces a strong response from normal animal immune systems. It has also been implicated in nonpathogenic aspects of bacterial ecology, including surface adhesion, bacteriophage sensitivity, and interactions with predators such as amoebae.
• Now let us discuss the structure of LPS. LPS is composed of two major parts, one is sugar and another is a lipid.
• Sugar part is a repetitive glycan polymer contained within an LPS is referred to as the O antigen, O polysaccharide, or O sidechain of the bacteria. The composition of the O chain varies from strain to strain. For example, there are over 160 different O antigen structures produced by different E. coli strains.
• The presence or absence of O chains determines whether the LPS is considered rough or smooth. Fulllength Ochains would render the LPS smooth, whereas the absence or reduction of Ochains would make the LPS rough.
• Bacteria with rough LPS usually have more penetrable cell membranes to hydrophobic antibiotics, since a rough LPS is more hydrophobic. In smooth LPS, O antigen is exposed on the very outer surface of the bacterial cell, and, as a consequence, is a target for recognition by host antibodies.
• The Core domain always contains an oligosaccharide component that attaches directly to lipid A and commonly contains sugars such as heptose and 3DeoxyDmannooct2ulosonic acid (also known as KDO, ketodeoxyoctulosonate).
• LPS acts as the prototypical endotoxin because it binds the CD14/TLR4/MD2 receptor complex in many cell types, but especially in monocytes, dendritic cells, macrophages, and B cells, which promotes the secretion of proinflammatory cytokines.
• Being of crucial importance to Gramnegative bacteria, these molecules make candidate targets for new antimicrobial agents.
• The molecular mimicry of some LPS molecules is thought to cause autoimmunebased host responses. Molecular mimicry is defined as the theoretical possibility that sequence similarities between foreign and selfpeptides are sufficient to result in the crossactivation of autoreactive immune cells by pathogenderived molecules.
Support my work at https://www.patreon.com/user?u=37177596
Twitter: https://twitter.com/DrKumarLokender
Facebook: https://www.facebook.com/lokenderkumar.sharma
Linkedin: https://www.linkedin.com/in/drlokenderkumar58525945/
Disclaimer: The information provided is for educational purposes only. The content of this channel should not be considered as medical advice of any kind. Please consult your doctor for medical help. Use this information at your own risk. We hold no responsibility for any issue, concerns, or damage arising from the content of the video. Under no circumstances Basic Science Series English be responsible or liable in any way for any content, including but not limited to, any errors or omissions in the content, any loss, any damage of any kind incurred as a result of any content communicated in this video, whether by Basic Science Series English or a third party. In no event shall Basic Science Series English be liable for any special indirect or consequential damages of any damages whatsoever resulting from the content of our channel.
New FDA Expectations for Endotoxin Testing
In January of last year, FDA released the guidance document Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile. Although the title of this document indicates that it is for sterility testing, much of the information is dedicated to endotoxin testing.
Specifically, this document established new expectations for implants, in addition to devices, that are labeled as nonpyrogenic, or that directly or indirectly contact the cardiovascular system, the lymphatic system, or cerebrospinal fluid. Because of this new guidance document, implant manufacturers whose 510(k)approved devices contact these areas may need to prepare to update their 510(k) clearance.
Historically, implants were exempt from USP 161 Transfusion and Infusion Assemblies and Similar Medical Devices, so endotoxin testing was not previously required. FDA now expects the following information to be included in the submission: a description of the method used, the endotoxin limit, an explanation supporting the limit, and a statement confirming that endotoxin testing will be done on every batch or a sampling plan. This webinar will include a review of the regulatory expectations for endotoxin testing, endotoxin limits, test methods, and example sampling plans. The information presented will be beneficial for medical device manufactures and QC professionals.
This webinar will include:
• regulatory expectations for endotoxin testing
• endotoxin limits
• test methods
• sampling plans
Endotoxin testing with the Endosafe® nexgen-MCS™
The Endosafe® nexgenMCS™ is a multicartridge benchtop endotoxin detection system that utilizes FDAlicensed Endosafe cartridges for fast, quantitative, and accurate endotoxin results. This video will show the features of the nexgenMCS, how to prepare a sample and analyze and export the results.
For over 70 years, Charles River has provided essential products and services to help pharmaceutical and biotechnology companies, government agencies, and leading academic institutions around the globe, helping to accelerate their research and drug development efforts. With over 90 sites globally, we’re ready to tackle your product’s most unique challenges. Our focus on timeliness and accuracy in every stage of drug development means you can count on us to deliver reliable results – every step of the way.
Learn more here: https://bit.ly/2ZLLz8p

นอกจากการดูหัวข้อนี้แล้ว คุณยังสามารถเข้าถึงบทวิจารณ์ดีๆ อื่นๆ อีกมากมายได้ที่นี่: ดูบทความเพิ่มเติมในหมวดหมู่GENERAL NEWS
Articles compiled by CASTU. See more articles in category: GENERAL NEWS


can you buy viagra over the counter how long does viagra take to kick in is viagra a controlled substance